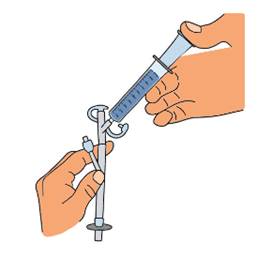
TIGLUTIK is the only formulation of riluzole approved for use with a PEG tube. Both silicone and polyurethane PEG tubes can be used.1 TIGLUTIK conveniently allows patients to get their prescribed dose of riluzole, and it eliminates the risk of crushed tablets clogging their feeding tube. TIGLUTIK is administered by placing the tip of the oral syringe containing TIGLUTIK 10 mL into the PEG tube. The plunger is slowly pushed until the oral syringe is empty.
A national survey of 887 people with ALS shows that 26% of patients have a PEG tube.2 Before the approval of TIGLUTIK, patients treated with riluzole had no other option but to crush their riluzole tablets. Crushed tablets are the most common cause of PEG tube occlusion occurring in up to 35% of patients.3 Clogged PEG tubes can lead to dehydration, malnutrition, and additional surgery.4 TIGLUTIK aligns with recommendations that patients with enteral feeding tubes should use liquid formulations of medication whenever possible.3
Click here for information to How to Order TIGLUTIK for Your Patients to ensure your patients with ALS get the full dose of riluzole they deserve.
Reference
- TIGLUTIK® (riluzole) [package insert]. Berwyn, PA: ITF Pharma; March 2020.
- The Voice of the Patient Report for Amyotrophic Lateral Sclerosis (ALS). Version Date: October 24, 2019. 1300 Wilson Boulevard, Suite 600Arlington, VA 22209.
- Blumenstein I, Neurologia. 2019;34(9):582-588.
- Brooks BR, Clinical Therapeutics. 2019;41(12);2490-2499.