TIGLUTIK is an oral suspension developed for people with ALS who have dysphagia. TIGLUTIK has a mildly thick consistency (IDDSI criteria Level 2).1 It was developed to help overcome the challenges that many patients have swallowing tablets. TIGLUTIK is administered by placing the tip of the oral syringe containing TIGLUTIK 10 mL inside the patient’s cheek. Slowly press the plunger until the oral syringe is empty.
Approximately 85% of people with ALS develop dysphagia caused by deficits of oropharyngeal motor and sensory neurons.2 For many patients, swallowing pills becomes difficult and can lead to significant health implications, including aspirating on pills.3 Before the availability of TIGLUTIK, patients who had difficulty swallowing pills were frequently advised to crush their riluzole tablets. However, no studies appear in medical literature showing that crushing riluzole tablets is safe to do.2 In some patients, crushing riluzole tablets may exacerbate dysphagia due to a laryngeal sensory deficit.2
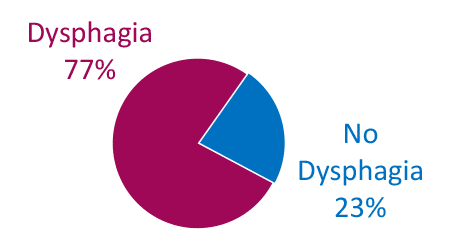
Dysphagia is Common in ALS
Bulbar Onset
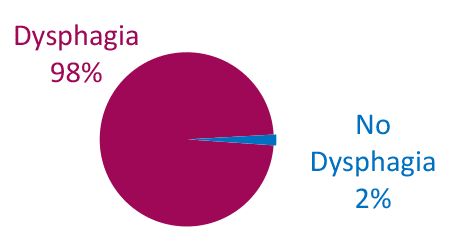
Limb Onset
Combined*
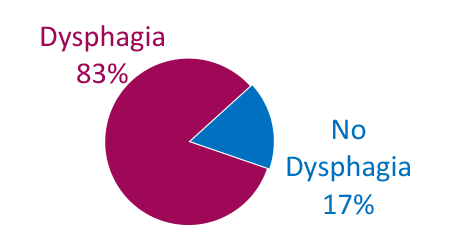
* Weighted average
Adapted from Onesti E. Frontiers in Neurology 2017;8:94.
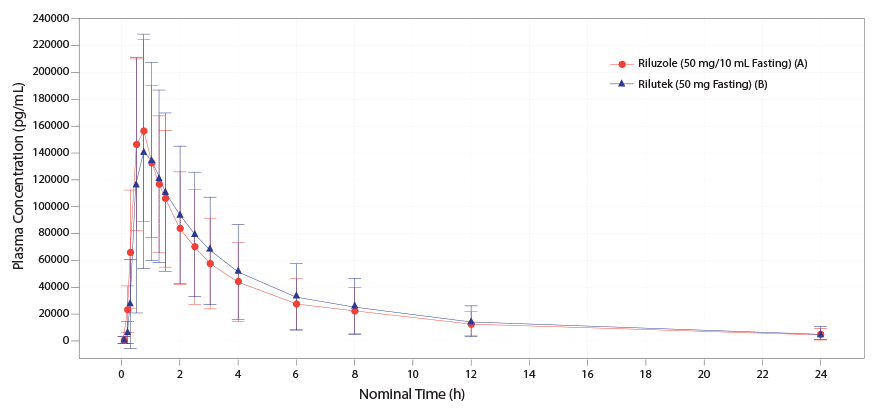
TIGLUTIK oral suspension 50 mg (10 mL) is bioequivalent to Rilutek® (riluzole) 50 mg tablets.4-6
Mean TIGLUTIK 50 mg (10 mL) is bioequivalent to Rilutek 50 mg tablets.
Click here for information to How to Order TIGLUTIK for Your Patients to ensure your patients with ALS get the full dose of riluzole they deserve.
References
- International Dysphagia Diet Standardisation Initiative. https://iddsi.org/. Accessed July 1, 2020.
- Onesti E, Front Neurol. 2017;8:94.
- Nativ-Zeltzer N, Front. Surg. 2019;6:43.
- TIGLUTIK® (riluzole) [package insert]. Berwyn, PA: ITF Pharma; March 2020.
- Rilutek (riluzole) [package insert]. Cary, NC: Covis Pharmaceuticals, Inc.; July 2016.
- Data on file. ITF Pharma. Berwyn, PA; December 2019.