Many people with ALS develop difficulty swallowing and are at risk of not getting adequate nutrition, becoming dehydrated, and not regularly taking their medications. Additionally, challenges with swallowing can lead to choking or foreign particles going into the lungs called aspiration. Aspiration can lead to a dangerous infection called aspiration pneumonia. If a patient or their caregiver believes aspiration occurred, they should contact their doctor as soon as possible.
Symptoms of Aspiration
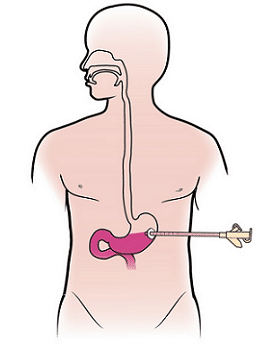
Approximately 1 out of 4 people with ALS have a Percutaneous Endoscopic Gastrostomy or PEG tube. A PEG tube is a permanent opening outside the person’s body into their stomach. They are about the diameter of a pencil or smaller and allows patients to receive adequate nutrition, fluids, and medication without swallowing. PEG tubes play a vital role in decreasing the risk of aspirating on food, beverages, and medications.
Patients should discuss with their physician the placement of PEG tubes soon after being diagnosed. Ideally, PEG tube placement should occur before swallowing symptoms begin to reduce nutritional deficiencies and the risk of aspiration. One of the reasons is that the progression of breathing muscle weakness usually occurs at the same time swallowing weakness does. When the ability to breathe is strong, the placement of these tubes is safer.
A PEG tube is an alternative way of eating and drinking, but this does not affect eating by mouth. PEG tubes supplement oral intake when swallowing weakness begins. The PEG tube becomes the sole method of nutrition, hydration, and taking medications if swallowing foods and fluids become too difficult or too dangerous.