When a foreign object like food goes into the lungs, it is called aspiration. Foreign objects can quickly lead to a lung infection and cause aspiration pneumonia. Aspiration pneumonia can be dangerous if left untreated. It is one of the leading causes of hospitalization and the second leading cause of death in ALS.
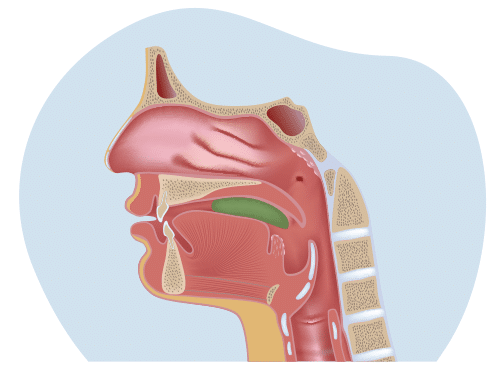
Dysphagia contributes to malnutrition, dehydration, and aspiration pneumonia. Together, these account for 1 out of 4 deaths in ALS. The risk of aspiration pneumonia is one of the main reasons patients should tell their doctor if they have difficulty swallowing. Too often, doctors don’t learn their patients had difficulty swallowing until being admitted to the hospital with aspiration pneumonia. Doctors need to intervene as early as possible if a patient has difficulty swallowing – before a patient has their first episode of aspiration.
Dysphagia also affects safely swallowing tablets. In people without swallowing problems, it is estimated that 7% of all foreign objects that are aspirated are caused by tablets. It is unknown how many people with ALS and who have dysphagia aspirate on tablets. Oral suspension formulations of medicines should be used instead of crushing tablets in people with dysphagia.
TIGLUTIK is an oral suspension of the medicine riluzole. It was specifically developed to meet the needs of people with ALS and to eliminate the need of crushing riluzole tablets. One dose of TIGLUTIK 50 mg (10 mL) (2 teaspoons) is equivalent to one RILUTEK® (riluzole) 50 mg tablet and it is taken twice a day, every 12 hours.
The EAT-10 is the Eating Assessment Tool and can help identify patients who have difficulty swallowing (dysphagia). It consists of 10 questions and is easy to take and score. It has been studied in people with ALS and shown to have “excellent accuracy” in identifying patients who aspirate. An EAT-10 score of 3 or higher suggests that a person may be having difficulty swallowing, and they should discuss their results with their doctor. The EAT-10 helps doctors better understand if their patients are having difficulty swallowing. Results from the EAT-10 is not equivalent to a clinical swallowing evaluation or instrumental assessment – only your doctors can determine this.
Click here for a copy of the EAT-10 questionnaire. After you print out the EAT-10, complete the 10 questions before your doctor’s appointment. Take the completed EAT-10 to your appointment and discuss the results with your doctor.

